ZusammensetzungWirkstoffe
1 Flasche mit Trockensubstanz
|
Wilate
|
500
|
1000
| |
Wirkstoffe:
| |
Gerinnungsfaktor VIII
|
500 I.E.*
|
1000 I.E.*
| |
Von-Willebrand-Faktor
|
500 I.E.**
|
1000 I.E.**
| |
(Gesamtproteingehalt)
|
(≤7,5 mg)
|
(≤15,0 mg)
|
* International Standard for Human Coagulation Factor VIII Concentrate.
** International Standard for von Willebrand Factor Concentrate.
Nach Auflösung mit der beigepackten Menge an Lösungsmittel enthält die Lösung 100 I.E. FVIII/100 I.E. VWF pro ml.
Hilfsstoffe
1 Flasche mit Trockensubstanz
|
Wilate
|
500
|
1000
| |
Hilfsstoffe:
| |
Natriumcitrat
|
14,7 mg
|
29,4 mg
| |
Natriumchlorid
|
117 mg
|
234 mg
| |
Kalziumchlorid
|
0,8 mg
|
1,5 mg
| |
Glycin
|
50 mg
|
100 mg
| |
Saccharose
|
50 mg
|
100 mg
| |
Lösungsmittel:
| |
Wasser für Injektionszwecke enthaltend 0.1% Polysorbat 80
|
5 ml
|
10 ml
|
Wilate 500 enthält bis zu 2,55 mmol (58,7 mg) Natrium und Wilate 1000 enthält bis zu 5,1 mmol (117,3 mg) Natrium pro Durchstechflasche.
Indikationen/AnwendungsmöglichkeitenVon-Willebrand-Syndrom
Vorbeugung und Behandlung von Blutungen oder Behandlung von Blutungen bei chirurgischen Eingriffen bei Patienten mit von-Willebrand-Syndrom (VWS), wenn die Behandlung mit Desmopressin (DDAVP) allein unwirksam oder kontraindiziert ist.
Hämophilie A
Therapie und Prophylaxe von Blutungen bei Patienten mit Hämophilie A (angeborener Faktor-VIII-Mangel).
Dosierung/AnwendungDas Präparat ist nur bei intravenöser Verabreichung wirksam.
Die Behandlung sollte nur unter Aufsicht eines in der Behandlung von Blutgerinnungsstörungen erfahrenen Arztes eingeleitet werden. Die Arzneimittel sind zum einmaligen Gebrauch bestimmt und der gesamte Inhalt sollte verwendet werden. Nicht verwendete Arzneimittel sind entsprechend den nationalen Anforderungen zu entsorgen.
Um die Rückverfolgbarkeit biologischer Arzneimittel sicherzustellen, wird empfohlen, Handelsname und Chargennummer bei jeder Behandlung zu dokumentieren.
Von-Willebrand-Syndrom
Das Verhältnis von FVIII-Aktivität zu VWF-Ristocetin-Kofaktor-Aktivität ist 1:1. Generell erhöht eine Einheit FVIII:C und VWF:RCo/kg KG die Aktivität um 1,5-2 I.E./dl des jeweiligen Proteins. Normalerweise werden ca. 20-50 I.E. Wilate/kg KG gegeben, um eine ausreichende Hämostase zu erreichen. Dies erhöht den FVIII:C und VWF:RCo im Patienten um ca. 30-100%.
Als Initialdosis können 50-80 I.E. Wilate/kg KG nötig sein, insbesondere bei Patienten mit von-Willebrand-Syndrom Typ 3, bei denen zur Erhaltung eines ausreichenden Plasmaspiegels höhere Dosierungen nötig sein können als bei anderen Typen des von-Willebrand-Syndroms.
Kinder und Jugendliche
Zur Anwendung von Wilate bei Kindern unter 6 Jahren liegen keine ausreichenden Daten vor.
Prävention von Blutungen bei Operationen und schweren Traumata:
Zur Prävention von Blutungen im Rahmen von Operationen sollte die Gabe von Wilate 1-2 Stunden vor dem Eingriff erfolgen.
Es sollten VWF:RCo-Plasmaspiegel von ≥ 60 I.E./dl (≥ 60 %) und FVIII:C-Plasmaspiegel von ≥ 40 I.E./dl (≥ 40 %) erreicht werden.
Während der Behandlung sollte eine entsprechende Dosis alle 12-24 Stunden gegeben werden. Die Dosierung und die Dauer der Therapie richten sich nach der klinischen Wirksamkeit, der Art und der Schwere der Blutung, sowie nach den VWF:RCo- und FVIII:C- Plasmaspiegeln.
Bei Verwendung eines FVIII-haltigen VWF-Präparates sollte der behandelnde Arzt berücksichtigen, dass Patienten mit von-Willebrand-Syndrom, die über einen längeren Zeitraum mit FVIII-haltigen VWF-Produkten behandelt werden, deutlich erhöhte FVIII:C-Plasmaspiegel aufweisen können. Diese können das Risiko einer Thrombose erhöhen, insbesondere bei Patienten mit bekannten klinischen und labordiagnostischen Risikofaktoren. Im Falle sehr hoher FVIII:C-Plasmaspiegel sollte eine Dosisreduzierung und/oder Verlängerung des Dosierungsintervalls bzw. der Einsatz von VWF-Präparaten mit niedrigem FVIII-Gehalt erwogen werden.
Vorbeugende Dauerbehandlung (Prophylaxe):
Für eine Langzeitprophylaxe bei VWS-Patienten sind 20 - 40 I.E. Wilate pro kg Körpergewicht zwei- oder dreimal wöchentlich zu verabreichen. In manchen Fällen, zum Beispiel bei Patienten mit gastrointestinalen Blutungen, können höhere Dosen erforderlich sein.
Hämophilie A
Dosierung
Dosis und Dauer der Therapie hängen vom Schweregrad des Faktor-VIII-Mangels sowie vom Ort und Ausmass der Blutung und dem klinischen Zustand des Patienten ab.
Die Anzahl der zu verabreichenden Faktor-VIII-Einheiten ist in Internationalen Einheiten angegeben (I.E.), die sich auf den derzeitigen WHO- Konzentrat-Standard für Faktor-VIII-Produkte beziehen. Die Faktor-VIII-Aktivität im Plasma wird entweder in Prozentsatz (bezogen auf normales Humanplasma) oder in Internationalen Einheiten (bezogen auf einen Internationalen Standard für Faktor-VIII im Plasma) angegeben. Eine Internationale Einheit (I.E.) der Faktor-VIII-Aktivität entspricht der FVIII-Menge in 1 ml normalem Humanplasma.
Bedarfsbehandlung:
Die Berechnung der erforderlichen Dosis an Faktor VIII basiert auf dem empirischen Befund, dass 1 Internationale Einheit (I.E.) Faktor-VIII vom Menschen pro kg Körpergewicht die Faktor-VIII-Aktivität im Plasma um 1,5% bis 2% der normalen Aktivität erhöht.
Die notwendige Dosis kann mit folgender Formel bestimmt werden:
Erforderliche Einheiten = Körpergewicht (kg) x gewünschter Faktor-VIII-Anstieg (%) (I.E./dl) x 0,5 I.E./kg
Die zu verabreichende Menge und die Häufigkeit der Verabreichung sollten sich im Einzelfall immer an der klinischen Wirksamkeit orientieren.
Im Falle der aufgeführten hämorrhagischen Ereignisse sollte die Faktor-VIII-Aktivität in dem entsprechenden Zeitraum nicht unter den angegebenen Plasmaspiegel (in % von normal oder I.E./dl) sinken.
Die nachfolgende Tabelle kann als Richtlinie zur Festlegung der Dosis bei Blutungsepisoden und chirurgischen Eingriffen dienen:
|
Blutungsgrad / Art der chirurgischen Intervention
|
Notwendiger
Faktor-VIII-Spiegel (%) (I.E./dl)
|
Behandlungshäufigkeit (Stunden) und -dauer (Tage)
| |
Blutungen
| |
Frühstadium von Gelenk- und Muskelblutungen, oder Blutungen im Mund
|
20 – 40
|
Infusion alle 12 - 24 Stunden wiederholen. Für mindestens 1 Tag bzw. bis die durch Schmerzen angezeigte Blutung sistiert oder eine Heilung erreicht ist.
| |
Grössere Blutungen, Gelenkblutungen, Muskelblutungen oder Hämatome
|
30 – 60
|
Infusion alle 12 - 24 Stunden wiederholen. Für 3 - 4 Tage oder länger, bis Schmerzen und gegebenenfalls Bewegungseinschränkungen beseitigt sind.
| |
Lebensbedrohliche Blutungen
|
60 – 100
|
Infusion alle 8 - 24 Stunden wiederholen, bis die Bedrohung abgewendet ist.
| |
Operationen
| |
Kleine Eingriffe (einschliesslich Zahnextraktionen)
|
30 – 60
|
Alle 24 Stunden, für mindestens 1 Tag bzw. bis Heilung erfolgt.
| |
Grosse Operationen
|
80 – 100
(prä- und postoperativ)
|
Infusion alle 8 - 24 Stunden wiederholen bis adäquate Wundheilung erfolgt. Anschliessend noch weitere 7 Tage therapieren, um einen Faktor-VIII-Spiegel von 30%-60% (I.E./dl) aufrecht zu erhalten.
|
Vorbeugende Dauerbehandlung (Prophylaxe):
Bei der Langzeitprophylaxe von Blutungen bei Patienten mit schwerer Hämophilie A betragen die üblichen Dosen 20 bis 40 I.E. Faktor VIII/kg Körpergewicht in Intervallen von 2 bis 3 Tagen. In manchen Fällen, besonders bei jüngeren Patienten, können kürzere Dosierungsintervalle oder höhere Dosen erforderlich sein.
Kontinuierliche Infusion:
Vor Operationen sollte eine pharmakokinetische Analyse durchgeführt werden, um die Clearance abschätzen zu können. Die initiale Infusionsrate kann folgendermassen errechnet werden:
Infusionsrate (I.E./kg/h) = Clearance (ml/kg/h) x gewünschter Spiegel (I.E./ml)
Nach den ersten 24 Stunden der kontinuierlichen Infusion sollte die Clearance täglich unter Verwendung der o.g. Gleichung mit dem gemessenen Wert und der verwendeten Infusionsrate errechnet werden.
Kinder und Jugendliche
Zur Anwendung von Wilate bei Kindern unter 6 Jahren sind bisher keine ausreichenden Daten vorhanden.
Art der Anwendung
Nach Auflösen wie unter «Sonstige Hinweise, Hinweise für die Handhabung» beschrieben, ist Wilate intravenös mit geringer Geschwindigkeit zu verabreichen. Die Injektions- bzw. Infusionsrate sollte 2-3 ml pro Minute nicht überschreiten.
KontraindikationenWilate darf nicht angewendet werden bei Überempfindlichkeit gegenüber einem der Wirkstoffe oder einem der Hilfsstoffe gemäss Zusammensetzung.
Warnhinweise und VorsichtsmassnahmenÜberempfindlichkeit
Allergische Überempfindlichkeitsreaktionen sind unter Wilate möglich. Falls Symptome einer Überempfindlichkeit auftreten, sollte den Patienten geraten werden, die Anwendung des Arzneimittels sofort abzubrechen und ihren Arzt aufzusuchen.
Patienten sollten über die Zeichen von Überempfindlichkeit oder allergische Reaktionen (wie z.B. Ausschlag, generalisierte Urtikaria, Engegefühl in der Brust, Stridor, Hypotonie bis hin zum anaphylaktischen Schock) aufgeklärt werden.
Beim Auftreten allergischer Symptome sollen Patienten die Behandlung sofort abbrechen und einen Arzt aufsuchen. Die aktuellen medizinischen Richtlinien zur Schocktherapie sind zu beachten.
Infektionserreger
Zur Prävention von Infektionen, die durch die Verwendung von Arzneimitteln aus humanem Blut oder Plasma übertragen werden können, werden Standardmassnahmen ergriffen. Diese Massnahmen beinhalten Auswahl der Spender, Testung der Einzelspenden und des Plasmapools auf spezifische Infektionsmarker und effektive Verfahrensschritte zur Inaktivierung/Entfernung von Viren.
Trotzdem kann die Möglichkeit der Übertragung infektiöser Agenzien bei der Anwendung von Arzneimitteln, die aus menschlichem Blut oder Plasma hergestellt wurden, nicht völlig ausgeschlossen werden. Dies gilt auch für unbekannte oder neu auftretende Viren oder andere Infektionserreger.
Die Massnahmen werden als wirksam für umhüllte Viren wie Humanes Immundefizienz-Virus (HIV), Hepatitis-B-Virus (HBV) und Hepatitis-C-Virus (HCV) und für das nicht umhüllte Hepatitis-A-Virus (HAV) angesehen.
Die Massnahmen können von begrenzter Wirksamkeit gegen nicht umhüllte Viren wie Parvovirus B19 sein.
Eine Parvovirus B19-Infektion kann für schwangere Frauen (Infektion des Fetus) und Patienten mit Immunschwäche oder mit gesteigerter Produktion von roten Blutkörperchen (z.B. bei hämolytischer Anämie) schwerwiegende Folgen haben.
Für Patienten, die regelmässig/wiederholt VWF-/Faktor-VIII-Präparate aus humanem Plasma erhalten, ist eine geeignete Impfung (Hepatitis A und Hepatitis B) in Betracht zu ziehen.
Bei jeder Verabreichung von Wilate müssen der Name und die Chargennummer des Präparates dokumentiert werden, um die Chargennachverfolgung zum Patienten zu gewährleisten.
Von-Willebrand-Syndrom
Thromboembolische Ereignisse
Bei Verwendung eines FVIII-haltigen VWF-Präparates sollte der behandelnde Arzt berücksichtigen, dass eine über längere Zeit fortgesetzte Therapie zu einem übermässig hohen Anstieg von FVIII:C führen kann. Bei Patienten, die FVIII-haltige VWF-Präparate erhalten, sollte daher der FVIII:C - Plasmaspiegel überwacht werden, um anhaltend übermässig hohe Spiegel zu vermeiden, die mit einem erhöhten Thromboserisiko einhergehen können.
Das Auftreten von thrombotischen Komplikationen, insbesondere bei Patienten mit bekannten klinischen oder labordiagnostisch nachgewiesenen Risikofaktoren, ist für FVIII-haltige VWF-Präparate beschrieben. Daher sind Patienten mit Risikofaktoren auf frühe Zeichen einer Thrombose zu überwachen. Generell sollte eine Thrombosephrophylaxe entsprechend den aktuellen Empfehlungen durchgeführt werden.
Inhibitoren
Patienten mit von-Willebrand-Syndrom, speziell Typ 3, können neutralisierende Antikörper (Inhibitoren) gegen VWF bilden. Falls der erwartete Anstieg des VWF:RCo-Plasmaspiegels nicht erreicht wird, oder falls die Blutung mit einer entsprechenden Dosis nicht kontrolliert werden kann, sollte ein Test auf VWF-Antikörper durchgeführt werden. Bei Patienten mit hochtitrigem Inhibitor kann die VWF-Therapie ineffektiv sein, und andere therapeutische Möglichkeiten sollten in Betracht gezogen werden. Die Behandlung solcher Patienten sollte durch Ärzte erfolgen, die Erfahrung in der Behandlung von Blutgerinnungsstörungen haben.
Hämophilie A
Die Bildung von neutralisierenden Antikörpern (Inhibitoren) gegen FVIII ist eine bekannte Komplikation bei der Behandlung von Hämophilie-A-Patienten. Diese Inhibitoren sind stets gegen die prokoagulatorische Aktivität von Faktor VIII gerichtete IgG-Immunglobuline, die in Bethesda-Einheiten (B.E.) pro ml Plasma mittels eines modifizierten Assays quantifiziert werden. Das Risiko, Inhibitoren zu entwickeln, korreliert mit dem Schweregrad der Erkrankung sowie der Exposition gegenüber dem Faktor VIII, wobei dieses Risiko innerhalb der ersten 50 Expositionstage am grössten ist, aber während des gesamten Lebens bestehen bleibt, auch wenn es nur gelegentlich auftritt.
Die klinische Relevanz der Inhibitorentwicklung ist abhängig vom Titer des Inhibitors, wobei niedrigtitrige Inhibitoren ein geringeres Risiko eines ungenügenden klinischen Ansprechens aufweisen als solche mit hohem Titer.
Ganz allgemein sollten alle Patienten, die mit Blutgerinnungsfaktor VIII behandelt wurden, sorgfältig mittels klinischer Befunde und mit geeigneten Labortests hinsichtlich der Entwicklung von Inhibitoren überwacht werden. Wenn der erwartete Faktor-VIII-Spiegel nicht erreicht wird oder die Blutung nicht durch die Verabreichung einer geeigneten Dosis gestillt werden kann, sollte der Patient auf Faktor-VIII-Hemmkörper hin untersucht werden. Bei Patienten mit hohen Inhibitorspiegeln kann die Faktor-VIII-Therapie unwirksam sein und es müssen andere Therapiemöglichkeiten in Betracht gezogen werden. Die Behandlung solcher Patienten sollte durch Ärzte erfolgen, die Erfahrung mit Hämophilie und mit Inhibitoren gegen Faktor-VIII haben.
Kardiovaskuläre Ereignisse
Bei Patienten mit bestehenden kardiovaskulären Risikofaktoren kann eine Substitutionstherapie mit FVIII das kardiovaskuläre Risiko erhöhen.
Katheter-assoziierte Komplikationen
Wenn ein zentraler Venenkatheter (ZVK) erforderlich ist, sollte das Risiko ZVK-assoziierter Komplikationen, einschliesslich lokaler Infektionen, Bakteriämie und Thrombosen an der Katheterstelle berücksichtigt werden.
Wilate 500 enthält bis zu 2,55 mmol (58,7 mg) Natrium und Wilate 1000 enthält bis zu 5,1 mmol (117,3 mg) Natrium pro Durchstechflasche. Dies entspricht 2,94 % bzw. 5,87 % der von der WHO empfohlenen maximalen täglichen Natriumaufnahme von 2 g für einen Erwachsenen.
Kinder und Jugendliche
Die aufgeführten Warnhinweise und Vorsichtsmassnahmen gelten für Erwachsene, Kinder und Jugendliche.
InteraktionenEs sind keine Wechselwirkungen von Arzneimitteln, die humanen VWF und Faktor VIII enthalten, mit anderen Arzneimitteln bekannt.
Schwangerschaft, StillzeitReproduktionsstudien an Tieren wurden mit Faktor VIII/VWF nicht durchgeführt.
Von-Willebrand-Syndrom
Gesicherte Daten zur Anwendung bei schwangeren oder stillenden Frauen liegen nicht vor. Wilate sollte bei schwangeren oder stillenden Frauen mit von-Willebrand-Syndrom nur angewendet werden, wenn dies unbedingt erforderlich ist. Es sollte berücksichtigt werden, dass der Geburtsvorgang ein erhöhtes Blutungsrisiko bei Frauen mit von-Willebrand-Syndrom darstellt.
Hämophilie A
Aufgrund des seltenen Auftretens von Hämophilie A bei Frauen liegen keine gesicherten Daten über die Anwendung während der Schwangerschaft und Stillzeit vor. Daher sollte Wilate bei schwangeren oder stillenden Frauen nur angewendet werden, wenn dies unbedingt erforderlich ist.
Wirkung auf die Fahrtüchtigkeit und auf das Bedienen von MaschinenWilate hat keinen Einfluss auf die Fahrtüchtigkeit oder die Fähigkeit, Maschinen zu bedienen.
Unerwünschte WirkungenÜberempfindlichkeit oder allergische Reaktionen (wie z.B. Angioödem, Brennen und Stechen an der Injektionsstelle, Schüttelfrost, Hitzegefühl, generalisierte Urtikaria, Erythem, Pruritus, Hautausschlag, Kopfschmerzen, Nesselsucht, Hypotonie, Lethargie, Übelkeit, nervöse Unruhe, Tachykardie, Engegefühl in der Brust, Dyspnoe, Kribbeln, Erbrechen, Stridor) wurden selten bei Patienten beobachtet, in Einzelfällen bis zur Ausbildung eines anaphylaktischen Schocks.
Die nachfolgende Tabelle gibt einen Überblick über Nebenwirkungen, die in klinischen Studien und in Studien zur Sicherheit nach Markteinführung beobachtet wurden, und solchen, die aus anderen Quellen nach Markteinführung stammen. Die Tabelle entspricht der MedDRA-Systemorganklassifizierung.
Die Häufigkeiten wurden gemäss folgender Konvention bestimmt:
sehr häufig (≥1/10)
häufig (≥1/100 bis <1/10)
gelegentlich (≥1/1'000 bis <1/100)
selten (≥1/10'000 bis <1/1'000)
sehr selten (<1/10'000)
nicht bekannt (Häufigkeit auf Grundlage der verfügbaren Daten nicht abschätzbar).
Bei spontan gemeldeten Nebenwirkungen nach Markteinführung wird die Häufigkeit als «nicht bekannt» eingestuft.
|
MedDRA Systemorganklasse
|
Nebenwirkung
|
Häufigkeit
| |
Erkrankungen des Immunsystems
|
Überempfindlichkeitsreaktionen (allergische Reaktionen)
|
Gelegentlich
| |
Anaphylaktischer Schock
|
Sehr selten
| |
Allgemeine Erkrankungen und Beschwerden am Verabreichungsort
|
Fieber
|
Gelegentlich
| |
Schmerzen im Brustraum
|
Nicht bekannt
| |
Erkrankungen des Blutes und des Lymphsystems
|
FVIII Inhibitoren
|
Gelegentlich (PTPs)*
Sehr häufig (PUPs)*
| |
VWF Inhibitoren
|
Sehr selten
| |
Erkrankungen der Atemwege, des Brustraums und Mediastinums
|
Husten
|
Nicht bekannt
| |
Erkrankungen des Nervensystems
|
Schwindel
|
Nicht bekannt
| |
Erkrankungen des Gastrointestinaltrakts
|
Bauchschmerzen
|
Nicht bekannt
| |
Skelettmuskulatur-, Bindegewebs- und Knochenerkrankungen
|
Rückenschmerzen
|
Nicht bekannt
|
* Die Häufigkeit basiert auf Studien mit allen FVIII-Produkten, wozu auch Patienten mit schwerer Hämophilie A gehörten. PTPs = vorbehandelte Patienten, PUPs = zuvor unbehandelte Patienten
Von Willebrand-Syndrom
Bei Patienten mit von Willebrand-Syndrom, speziell Typ 3 - Patienten, können in sehr seltenen Fällen Hemmkörper gegen den VWF entwickeln. Das Auftreten solcher Inhibitoren manifestiert sich in einer unzureichenden klinischen Wirksamkeit. Diese Hemmkörper können Komplexe bilden und können gleichzeitig mit anaphylaktischen Reaktionen auftreten. Deswegen sollten Patienten mit anaphylaktischen Reaktionen auf Hemmkörper getestet werden.
Es wird empfohlen, in diesen Fällen Kontakt mit einem auf Hämophilie spezialisierten Zentrum aufzunehmen.
Es besteht ein Risiko für thrombotische Ereignisse, besonders bei Patienten mit bekannten klinischen oder labordiagnostisch nachgewiesenen Risikofaktoren.
Eine Thromboseprophylaxe sollte entsprechend den aktuellen Empfehlungen eingeleitet werden.
Bei Patienten mit von-Willebrand-Syndrom, die FVIII-haltige VWF-Produkte erhalten, können anhaltend hohe FVIII:C-Plamaspiegel zu einem erhöhten Thromboserisiko führen.
Hämophilie A
Bei Patienten mit Hämophilie A, die mit Faktor VIII, einschliesslich Wilate, behandelt werden, können sich neutralisierende Antikörper (Inhibitoren) entwickeln. Bei Auftreten solcher wird sich dieser Zustand in einer unzureichenden klinischen Wirksamkeit manifestieren. Kontakt mit einem auf Hämophilie spezialisierten Zentrum aufzunehmen.
Zu Informationen zur Virussicherheit siehe «Warnhinweise und Vorsichtsmassnahmen».
ÜberdosierungSymptome von Überdosierung mit humanem Faktor VIII/VWF wurden bislang nicht beobachtet. Thromboembolische Komplikationen können bei massiver Überdosierung auftreten.
Eigenschaften/WirkungenATC-Code
B02BD06
Pharmakotherapeutische Gruppe: Anti-Hämorrhagikum, Blutgerinnungsfaktoren:
Von Willebrand-Faktor human und Faktor VIII human in Kombination
Wirkungsmechanismus
VWF (im Konzentrat) ist ein normaler Bestandteil des menschlichen Plasmas und verhält sich wie körpereigener VWF.
Faktor VIII (im Konzentrat) ist ein normaler Bestandteil des menschlichen Plasmas und verhält sich wie körpereigener Faktor VIII.
Pharmakodynamik
Der Faktor VIII/von-Willebrand-Faktor-Komplex besteht aus zwei Molekülen (Faktor-VIII und VWF) mit unterschiedlichen physiologischen Funktionen.
Von-Willebrand-Faktor
·VWF stellt die Plättchen-Adhäsion an das vaskuläre Sub-Endothelium an der Stelle der vaskulären Verletzung wieder her (da es an das vaskuläre Sub-Endothelium und an die Plättchenmembran bindet). VWF sorgt für die primäre Hämostase, was durch die Verkürzung der Blutungszeit sichtbar wird. Dieser Effekt tritt unmittelbar auf und hängt zum Grossteil vom Grad der Polymerisation des Proteins ab;
·VWF führt zur verzögerten Korrektur eines assoziierten Faktor-VIII-Mangels. Intravenös verabreichter VWF bindet an den endogenen Faktor VIII (der normalerweise vom Patienten produziert wird) und stabilisiert diesen, indem er eine rasche Degradation verhindert.
Neben seiner Schutzfunktion für den Faktor-VIII vermittelt der VWF die Adhäsion der Plättchen an einem Verletzungsort und spielt eine Rolle bei der Aggregation der Plättchen.
Faktor VIII
Aktivierter Faktor VIII wirkt als Kofaktor für aktivierten Faktor IX und beschleunigt die Umwandlung von Faktor X zu aktiviertem Faktor X (FXa). FXa wandelt Prothrombin zu Thrombin um. Thrombin wandelt dann Fibrinogen zu Fibrin um, wodurch ein Gerinnsel gebildet werden kann.
Bei der Behandlung hämophiler Patienten bindet infundierter Faktor VIII an den Von-Willebrand-Faktor im Blutkreislauf des Patienten.
Neben seiner Schutzfunktion für den Faktor VIII, vermittelt der Von-Willebrand-Faktor die Adhäsion der Plättchen dort, wo Gefässe verletzt sind und ist an der Aggregation der Plättchen beteiligt.
Klinische Wirksamkeit
Von Willebrand-Syndrom
Die Verabreichung von VWF erlaubt die Korrektur von Blutgerinnungsstörungen bei Patienten, die an VWF-Mangel leiden. Die Gabe von reinem VWF (VWF Präparat mit geringem Faktor VIII-Gehalt) stellt den FVIII:C-Plasmaspiegel nach der ersten Infusion erst mit Verzögerung wieder her.
Die Gabe eines FVIII:C-haltigen VWF-Präparates stellt den FVIII:C-Plasmaspiegel nach der ersten Infusion sofort wieder her.
Hämophilie A
Hämophilie A ist in der Regel eine geschlechtsbezogene erbliche Blutgerinnungsstörung, die auf verringerte Spiegel von FVIII:C zurückzuführen ist. Sie kann zu massiven Blutungen in Gelenken, Muskeln oder inneren Organen führen, die entweder spontan auftreten oder als Folge von Verletzungen oder chirurgischen Eingriffen.
Durch die Substitutionstherapie wird der Faktor-VIII-Spiegel im Plasma erhöht und somit ist eine vorübergehende Korrektur des Faktormangels und der Blutungstendenz herbeigeführt.
Zu beachten ist, dass die annualisierte Blutungsrate (ABR) zwischen verschiedenen Faktorkonzentraten und verschiedenen klinischen Studien nicht vergleichbar ist.
PharmakokinetikAbsorption
Der VWF (im Konzentrat) ist ein normaler Bestandteil des menschlichen Plasmas und verhält sich wie der körpereigene VWF.
Distribution
Siehe Elimination.
Metabolismus
Nicht zutreffend.
Elimination
Von-Willebrand-Syndrom
Basierend auf der Meta-Analyse von drei pharmakokinetischen Studien mit auswertbaren Daten von 24 Patienten (alle VWS-Typen) wurden die folgenden Ergebnisse beobachtet:
|
|
Alle VWS-Typen
|
VWS-Typ 1
| |
Parameter
|
N
|
Mittel
|
SD
|
Min.
|
Max.
|
N
|
Mittel
|
SD
|
Min.
|
Max.
| |
Recovery (%/IE/kg)
|
24
|
1,56
|
0,48
|
0,90
|
2,93
|
2
|
1,19
|
0,07
|
1,14
|
1,24
| |
AUC (0-inf) (h*%)
|
23
|
1981
|
960
|
593
|
4831
|
2
|
2062
|
510
|
1701
|
2423
| |
T ½ (h)
|
24
|
23,3
|
12,6
|
7,4
|
58,4
|
2
|
39,7
|
18,3
|
26,7
|
52,7
| |
MRT (h)
|
24
|
33,1
|
19
|
10,1
|
89,7
|
2
|
53,6
|
25,9
|
35,3
|
71,9
| |
Clearance (ml/h/kg)
|
24
|
3,29
|
1,67
|
0,91
|
7,41
|
2
|
2,66
|
0,85
|
2,06
|
3,27
|
|
|
VWS-Typ 2
|
VWS-Typ 3
| |
Parameter
|
N
|
Mittel
|
SD
|
Min.
|
Max.
|
N
|
Mittel
|
SD
|
Min.
|
Max.
| |
Recovery (%/IE/kg)
|
5
|
1,83
|
0,86
|
0,98
|
2,93
|
17
|
1,52
|
0,32
|
0,90
|
2,24
| |
AUC (0-inf) (h*%)
|
5
|
2971
|
1383
|
1511
|
4831
|
16
|
1662
|
622
|
593
|
2606
| |
T ½ (h)
|
5
|
34,9
|
16
|
17,5
|
58,4
|
17
|
18
|
6,2
|
7,4
|
30,5
| |
MRT (h)
|
5
|
53,5
|
24,6
|
27,8
|
89,7
|
17
|
24,7
|
8,5
|
10,1
|
37,7
| |
Clearance (ml/h/kg)
|
5
|
1,95
|
1,02
|
0,91
|
3,31
|
17
|
3,76
|
1,69
|
1,83
|
7,41
|
SD = Standardabweichung
AUC (area under the curve) = Fläche unter der Konzentrations-Zeit-Kurve bezogen auf das KG
T ½ = Halbwertzeit
MRT (mean residence time) = Mittlere Verweildauer
Hämophilie A
Faktor VIII (im Konzentrat) ist ein normaler Bestandteil des menschlichen Plasmas und verhält sich wie körpereigener Faktor VIII.
Nach Injektion des Produktes bleiben ungefähr 2/3 bis 3/4 des Faktor VIII im Blutkreislauf. Die Faktor-VIII-Aktivität im Plasma sollte zwischen 80%-120% der erwarteten Faktor-VIII-Aktivität liegen.
Die Faktor-VIII-Aktivität verringert sich exponentiell in zwei Phasen. In der initialen Phase wird der Faktor VIII zwischen dem Intravaskularraum und anderen Kompartimenten (Körperflüssigkeiten) verteilt und wird mit einer Halbwertzeit von 3 bis 6 Stunden aus dem Plasma entfernt. In der folgenden langsameren Phase variiert die Halbwertzeit zwischen 8 und 18 Stunden, mit einem Durchschnitt von 15 Stunden. Dies entspricht der biologischen Halbwertzeit.
Für Wilate wurden in einer Pharmakokinetikstudie die folgenden Resultate mit 12 Patienten ermittelt (chromogene Methode, Doppelbestimmung):
|
Parameter
|
Initialwerte
|
6-Monatswerte
| |
Mittelwert
|
SD
|
Mittelwert
|
SD
| |
Recovery
%/IE/kg
|
FVIII:C 2,27
|
1,20
|
FVIII:C 2,26
|
1,19
| |
AUCnorm
% * h/IU/kg
|
FVIII:C 31,3
|
7,31
|
FVIII:C 33,8
|
10,9
| |
Halbwertzeit (h)
|
FVIII:C 11,2
|
2,85
|
FVIII:C 11,8
|
3,37
| |
MRT (h)
|
FVIII:C 15,3
|
3,5
|
FVIII:C 16,3
|
4,6
| |
Clearance
ml/h/kg
|
FVIII:C
3,37
|
0,86
|
FVIII:C
3,24
|
1,04
|
SD = Standardabweichung
AUC (area under the curve) = Fläche unter der Konzentrations Zeit-Kurve bezogen auf das KG
MRT mean residence time = Mittlere Verweildauer
Präklinische DatenDie in Wilate enthaltenen arzneilich wirksamen Bestandteile FVIII und VWF sind normale Bestandteile des menschlichen Plasmas und verhalten sich wie körpereigene Faktoren.
Herkömmliche Tierversuche mit diesen Faktoren würden keine verwertbaren Informationen über die bestehenden klinischen Erfahrungen hinaus erbringen und sind somit nicht erforderlich.
Sonstige HinweiseInkompatibilitäten
Da keine Verträglichkeitsstudien durchgeführt wurden, darf dieses Arzneimittel nicht mit anderen Arzneimitteln gemischt oder gleichzeitig mit anderen intravenösen Zubereitungen im gleichen Schlauchsystem verabreicht werden.
Zur intravenösen Gabe soll ausschliesslich das beigefügte Injektions-/Infusionsset verwendet werden, da eine Adsorption von Faktor VIII/Von-Willebrand-Faktor an den Innenoberflächen anderer Injektions-/Infusionssets nicht ausgeschlossen werden kann.
Beeinflussung diagnostischer Methoden
Eine Beeinflussung diagnostischer Methoden ist nicht bekannt.
Haltbarkeit
3 Jahre.
Das Verfalldatum ist auf dem Behältnis und der Verpackung aufgedruckt. Wilate darf nach diesem Datum nicht mehr verwendet werden.
Das gebrauchsfertige Präparat soll unmittelbar nach dem Auflösen verwendet werden.
Die Haltbarkeit der gebrauchsfertigen Lösung wurde für 4 Stunden bei Raumtemperatur (bis max. 25°C) nachgewiesen. Aus mikrobiologischer Sicht ist die gebrauchsfertige Lösung jedoch umgehend nach Herstellung zu verwenden.
Besondere Lagerungshinweise
Pulver und Lösungsmittel bei + 2°C bis +8°C (im Kühlschrank) lagern und in der Originalverpackung aufbewahren, um den Inhalt vor Licht zu schützen. Nicht einfrieren.
Wilate kann auch bei Raumtemperatur (bis max. 25°C) 2 Monate aufbewahrt werden. In diesem Fall läuft die Haltbarkeit des Produktes 2 Monate nach der ersten Entnahme aus dem Kühlschrank ab. Das neue Haltbarkeitsdatum muss vom Patienten aussen auf dem Karton vermerkt werden.
Die gebrauchsfertige Lösung ist nur zum einmaligen Gebrauch bestimmt. Nicht verbrauchte Lösung verwerfen.
Arzneimittel sorgfältig und für Kinder unzugänglich aufbewahren!
Hinweise für die Handhabung
Zur intravenösen Injektion nach Auflösen im beigefügten Lösungsmittel (Wasser für Injektionszwecke mit 0.1% Polysorbat 80)
·Bitte lesen Sie alle Anweisungen durch und befolgen Sie sie sorgfältig.
·Verwenden Sie Wilate nicht mehr nach dem Verfalldatum, das auf dem Etikett und dem Umkarton angegeben ist.
·Bitte achten Sie bei allen Arbeitsschritten strikt auf Keimfreiheit.
·Das rekonstituierte Arzneimittel sollte vor der Verabreichung visuell auf Partikel und Verfärbungen überprüft werden.
·Die Lösung sollte klar oder leicht schillernd (opaleszent) sein. Verwenden Sie keine Lösungen, die trübe aussehen oder Rückstände enthalten.
·Das gebrauchsfertige Präparat unmittelbar nach dem Auflösen verwenden, um mikrobielle Verunreinigungen zu verhindern.
·Verwenden Sie bitte ausschliesslich das mitgelieferte Infusionszubehör. Die Verwendung anderer Injektions-/Infusionsbestecke kann mit Risiken verbunden sein oder die Wirksamkeit beeinträchtigen.
Anleitung für das Auflösen:
|
|
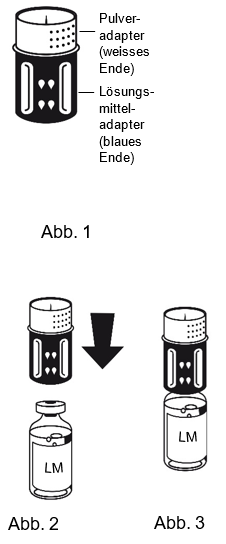
|
1. Lösungsmittel und Pulver in den ungeöffneten Flaschen auf Zimmertemperatur bringen. Nicht direkt aus dem Kühlschrank verwenden.
2. Die Schutzkappen von der Pulverflasche und Lösungsmittelflasche entfernen und die Gummistopfen beider Flaschen mit einem Alkoholtupfer desinfizieren.
3. Die Lösungsmittelflasche auf eine feste, ebene Fläche stellen. Das in Abb. 1 beschriebene Transferset mit dem blauen Adapter auf die Lösungsmittelflasche (LM) aufsetzen und bis zum Anschlag nach unten drücken (Abb. 2+3). Während des Aufsetzens das Transferset nicht drehen.
| |
|
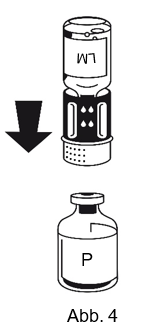
|
4. Die Pulverflasche (P) auf eine feste, ebene Fläche stellen. Die Lösungsmittelflasche (LM) mit dem Transferset umdrehen und senkrecht mit dem transparenten Ende auf die Pulverflasche (P) aufsetzen und bis zum Anschlag nach unten drücken (Abb. 4). Während des Aufsetzens das Transferset nicht drehen. Das Vakuum in der Pulverflasche saugt das Lösungsmittel an.
| |
|
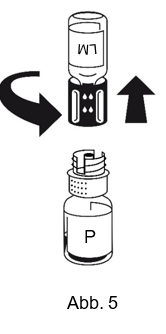
|
5. Die Pulverflasche mit Transferset und der verbundenen Lösungsmittelflasche leicht schwenken (nicht schütteln) bis das Pulver vollständig gelöst ist. Das Pulver löst sich bei Zimmertemperatur spätestens nach 10 Minuten vollständig. Dabei ist eine leichte Schaumbildung möglich, die sich auflösen wird. Das Transferset auseinander schrauben (Abb. 5). Die leere Lösungsmittelflasche zusammen mit dem blauen Transferset-Adapter entsorgen.
|
Injektion:
Der Puls sollte vor und während der Injektion gemessen werden. Eine deutliche Erhöhung der Pulsfrequenz klingt normalerweise nach Verlangsamen oder Unterbrechen der Injektion schnell wieder ab.
|
|
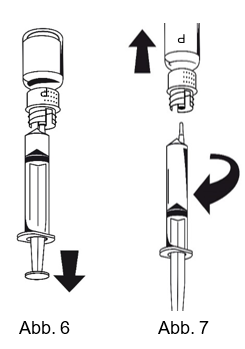
|
1. Die Spritze mit dem weissen Transferset-Adapter der Pulverflasche verbinden. Die Flasche samt Einmalspritze umdrehen und das aufgelöste Präparat in die Spritze aufziehen (Abb. 6).
Die Injektionslösung sollte klar oder leicht schillernd sein. Nachdem die Lösung in die Spritze überführt wurde, den Spritzenzylinder fassen und die Spritze vom weissen Transferset-Adapter der Pulverflasche entfernen (Abb. 7). Die leere Pulverflasche zusammen mit dem weissen Transferset-Adapter entsorgen.
|
2. Vorgesehene Injektionsstelle mit einem Alkoholtupfer desinfizieren.
3. Das beigepackte Infusionsset auf die Spritze aufsetzen.
4. Stechen Sie die Flügelkanüle in die gewählte Vene. Wenn Sie die Vene vor der Punktion gestaut haben, damit Sie sie besser sehen können, müssen Sie die Stauung öffnen, bevor Sie mit der Injektion beginnen. Es darf kein Blut in die Spritze gelangen, da dies zur Bildung von Blutgerinnseln führen könnte.
5. Injizieren Sie die Lösung langsam in die Vene, wobei die Injektionsgeschwindigkeit höchstens 2 - 3 ml pro Minute betragen sollte.
Wenn Sie mehr als eine Flasche Wilate für die Behandlung benötigen, kann die Injektionskanüle in der Vene belassen werden. Das Transferset ist nur zum einmaligen Gebrauch bestimmt.
Nicht verbrauchte Lösung und Abfallmaterialien müssen entsprechend den gültigen Vorschriften entsorgt werden.
Zulassungsnummer56133 (Swissmedic)
PackungenWilate 500 (500 I.E. FVIII und 500 I.E. VWF)
1 Packung enthält:
1 Durchstechflasche mit Pulver, Typ-I-Glas, verschlossen mit einem Brombutylstopfen und einer Flip-off-Bördelkappe
1 Durchstechflasche mit Lösungsmittel (5 ml Wasser für Injektionszwecke mit 0,1% Polysorbat 80)
1 Gerätesatz für intravenöse Injektion: 1 Transferset, 1 Flügelkanüle, 1 Einmalspritze
2 Alkoholtupfer (B)
Wilate 1000 (1000 I.E. FVIII und 1000 I.E. VWF)
1 Packung enthält:
1 Durchstechflasche mit Pulver, Typ-I-Glas, verschlossen mit einem Brombutylstopfen und einer Flip-off Bördelkappe
1 Durchstechflasche mit Lösungsmittel (10 ml Wasser für Injektionszwecke mit 0,1% Polysorbat 80)
1 Gerätesatz für intravenöse Injektion: 1 Transferset, 1 Flügelkanüle, 1 Einmalspritze
2 Alkoholtupfer (B)
ZulassungsinhaberinOCTAPHARMA AG, Seidenstrasse 2, CH-8853 Lachen
Stand der InformationMärz 2022
|




