ZusammensetzungWirkstoffe
Leuprorelini acetas.
Hilfsstoffe
Mannitolum, Acidum polylacticum (PLA).
Kammer mit dem Lösungsmittel (1 ml): Mannitolum, Carmellosum natricum corresp. Natrium max. 0.54 mg/ml, Polysorbatum 80, Acidum aceticum glaciale, Aqua ad injectabilia.
Indikationen/AnwendungsmöglichkeitenProstatakarzinom: Symptomatische Behandlung des fortgeschrittenen hormonabhängigen Prostatakarzinoms. Als alternative Behandlung, wenn Orchiektomie oder Östrogengaben entweder für den Patienten nicht indiziert oder nicht zumutbar sind.
Mammakarzinom: Adjuvante Therapie des frühen operablen Mammakarzinoms und Therapie des fortgeschrittenen, metastasierenden Mammakarzinoms bei prämenopausalen Frauen mit Rezeptor-positiven Tumoren, bei denen eine Hormontherapie angezeigt ist.
Endometriose: Symptomatische, laparoskopisch gesicherte Endometriose, wenn eine Unterdrückung der ovariellen Hormonbildung angezeigt ist, sofern die Erkrankung nicht primär einer chirurgischen Therapie bedarf.
Zur Reduktion der Östrogenmangelsymptome (einschliesslich Osteoporose – vgl. «Warnhinweise und Vorsichtsmassnahmen») wird eine add-back Therapie empfohlen (vgl. «Eigenschaften/Wirkungen»).
Dosierung/AnwendungDie Injektionsstelle sollte bei jeder Injektion gewechselt werden (Bauchhaut, Gesäss, Oberschenkel).
Zur Injektion wird eine Nadel von 23 Gauge (z.B. 0,6 x 25 mm) empfohlen.
Prostatakarzinom
Alle 12 Wochen 1 Zweikammerspritze zu 11,25 mg subkutan.
Die Anwendung von Lucrin Depot 3 Monate beim Prostatakarzinom sollte nur unter Überwachung eines in der Tumortherapie erfahrenen Arztes erfolgen. Für die Initialphase der Behandlung sollte die zusätzliche Gabe eines geeigneten Antiandrogens erwogen werden, um so die möglichen Folgeerscheinungen des anfänglichen Testosteronanstiegs und die vorübergehende Verschlechterung der klinischen Symptomatik abzuschwächen. Die Behandlung mit einem GnRH-Analogon bei Patienten, die an einem Prostatakarzinom erkrankt sind, wird üblicherweise auch nach Erreichen einer Kastrationsresistenz fortgeführt. Die relevanten Leitlinien sind zu beachten.
Mammakarzinom
Alle 12 Wochen 1 Zweikammerspritze zu 11,25 mg subkutan.
Für die adjuvante Therapie des Mammakarzinoms wurden klinische Daten mit Lucrin Depot 3 Monate für eine Behandlungsdauer von bis zu 24 Monaten erhoben. Bei der Festlegung der individuellen Behandlungsdauer sollen Nutzen und Verträglichkeit der Behandlung berücksichtigt werden. Bei Verdacht auf eine ungenügende hormonelle Suppression soll eine Östrogenbestimmung durchgeführt werden.
Eine Behandlungsdauer von mehr als 2 Jahren wurde nicht untersucht.
Endometriose
Alle 12 Wochen 1 Zweikammerspritze zu 11,25 mg intramuskulär.
Die erste Injektion sollte etwa am 3. Tag der Menstruation erfolgen, um eine bestehende Schwangerschaft auszuschliessen. Im Zweifelsfall wird die Durchführung eines Schwangerschaftstests empfohlen (siehe «Warnhinweise und Vorsichtsmassnahmen»).
Die Dauer der Anwendung ist auf einen Zeitraum von 6 Monaten zu begrenzen. Wiederholungsbehandlungen sollten nur nach sorgfältiger Nutzen-Risiko-Abwägung erfolgen. Dazu gehört die Bestimmung der Knochendichte vor Beginn einer eventuellen Wiederholungstherapie.
Spezielle Dosierungsempfehlungen
Kinder / Jugendliche: Die Wirksamkeit und Sicherheit von Lucrin Depot 3 Monate wurde bei Kindern und Jugendlichen nicht untersucht. Eine Behandlung in dieser Altersgruppe wird daher nicht empfohlen.
Ältere Patienten: Eine Dosisanpassung in Abhängigkeit vom Alter ist nicht erforderlich.
Leber- und Niereninsuffizienz: Eine Dosisanpassung ist nicht erforderlich (vgl. «Pharmakokinetik»).
KontraindikationenÜberempfindlichkeit gegenüber dem Wirkstoff, ähnlichen Nona- oder Dekapeptiden oder einem der Hilfsstoffe gemäss Zusammensetzung.
Anwendung bei Prostatakarzinom: Bei nachgewiesener Hormonunabhängigkeit des Prostatakarzinoms ist Lucrin nicht indiziert.
Anwendung bei Endometriose und Mammakarzinom: Lucrin Depot 3 Monate ist kontraindiziert bei schwangeren Frauen oder Frauen, welche möglicherweise schwanger werden könnten (siehe «Schwangerschaft/Stillzeit»).
Leuprorelinacetat darf im Falle nicht abgeklärter irregulärer Vaginalblutungen nicht verabreicht werden.
Warnhinweise und VorsichtsmassnahmenEine intraarterielle Injektion ist unbedingt zu vermeiden.
Hypophysenapoplexie:
Während der Marktüberwachung wurde nach der Verabreichung von Gonadotropin-Releasing-Hormon (GnRH)-Agonisten selten über Hypophysenapoplexie berichtet (sekundäres klinisches Syndrom eines Hypophysen-Infarktes). In den meisten dieser Fälle wurde ein Hypophysenadenom diagnostiziert. Die Mehrheit dieser Hypophysenapoplexie-Fälle traten innerhalb von 2 Wochen, einige innerhalb der ersten Stunde, nach Verabreichung der ersten Dosis auf. In diesen Fällen zeigte sich die Hypophysenapoplexie durch plötzliche Kopfschmerzen, Erbrechen, visuelle Veränderungen, Ophthalmoplegie, einen veränderten mentalen Status und manchmal einen kardiovaskulären Kollaps. Sofortige medizinische Betreuung war erforderlich.
Bei bekanntem Hypophysenadenom sollte aus diesem Grund ein GnRH-Agonist nicht gegeben werden.
Suizidrisiko:
Patienten mit vorbestehenden Depressionen können suizidgefährdet sein.
Knochendichte/Frakturrisiko:
Während eines hypo-östrogenen Zustands können Veränderungen der Knochendichte auftreten. Die Abnahme der Knochendichte kann nach Absetzen von Lucrin Depot 3 Monate reversibel sein.
Durch den Mangel an Androgenen, welcher aus der Behandlung des Prostatakarzinoms resultiert, kann das Frakturrisiko auch bei Männern erhöht sein.
Krampfanfälle:
Im Rahmen der Marktüberwachung wurde, insbesondere bei Frauen und Kindern, über das Auftreten von Krampfanfällen berichtet. Teilweise handelte es sich dabei um Patienten, welche andere Risikofaktoren für Krampfanfälle aufwiesen (wie z.B. Epilepsie-Anamnese, intrakranielle Tumoren, Komedikation mit Arzneimitteln, für welche ein Risiko für Krampfanfälle bekannt ist). Es liegen jedoch auch Meldungen von Patienten ohne derartige Risikofaktoren vor.
Verzögert auftretende Überempfindlichkeitsreaktionen:
Verzögerte Überempfindlichkeitsreaktionen, einschliesslich schweren kutanen unerwünschten Reaktionen (SCAR) des Stevens-Johnson-Syndroms (SJS) und der toxischen epidermalen Nekrolyse (TEN), wurden nach der Markteinführung sehr selten im Zusammenhang mit der Leuprorelinacetat-Therapie berichtet (siehe «Unerwünschte Wirkungen»). Bei den ersten Anzeichen oder Symptomen einer verzögerten Überempfindlichkeitsreaktion ist die weitere Behandlung mit Leuprorelinacetat abzubrechen, und die Patienten sind entsprechend der gängigen klinischen Praxis zu behandeln.
Stoffwechselveränderungen:
Die Anwendung einer Androgendeprivationstherapie (ADT), einschliesslich GnRH-Agonisten, kann mit einem erhöhten Risiko für Stoffwechselveränderungen wie Hyperglykämie, Diabetes, Hyperlipidämie und nicht-alkoholische Fettlebererkrankung (NAFLD) verbunden sein. Hyperglykämie kann die Entwicklung von Diabetes mellitus oder eine Verschlechterung der Blutzuckerkontrolle bei Patienten mit Diabetes bedeuten. Patienten die einen GnRH-Agonisten erhalten sollten auf die Anzeichen und Symptome des metabolischen Syndroms, einschliesslich der Lipide, des Blutzuckers und/oder des glykosylierten Hämoglobins (HbA1c), überwacht und gemäss der gängigen klinischen Praxis behandelt werden (siehe «Unerwünschte Wirkungen»).
Kardiovaskuläre Risikofaktoren/Erkrankungen:
Unter der Behandlung mit GnRH-Agonisten wurde über ein erhöhtes Risiko für Diabetes mellitus und/oder kardiovaskuläre Ereignisse berichtet. Insbesondere wurde in mehreren grossen epidemiologischen Studien bei Prostatakarzinom-Patienten ein um etwa 20 % erhöhtes Risiko für Myokardinfarkt und Schlaganfall beobachtet. Bei Patienten mit Vorliegen weiterer Risikofaktoren für kardiovaskuläre Ereignisse (z.B. Hypertonie, Hyperlipidämie) bzw. mit bereits bestehenden kardiovaskulären Erkrankungen sollte daher eine besonders sorgfältige Nutzen-Risiko-Abwägung erfolgen.
Vor Einleitung einer Behandlung mit Leuprorelin sollten Blutdruck, Blutglucose und Lipidprofil bestimmt werden. Während der Behandlung sollten die Patienten bezüglich dieser Risikofaktoren sowie bezüglich möglicher Symptome, welche die Entwicklung einer kardiovaskulären Erkrankung vermuten lassen, überwacht werden. Bereits bestehende Risikofaktoren (Diabetes, Hypercholesterinämie, Hypertonie) sollten adäquat, d.h. entsprechend der jeweiligen Guidelines, behandelt werden.
Effekte auf das QT-Intervall:
Eine Androgendeprivation kann das QT-Intervall verlängern. Bei Patienten mit einer Vorgeschichte oder einem Risiko für eine QT Verlängerung und bei Patienten, welche gleichzeitig Arzneimittel erhalten, die eine QT Verlängerung bewirken können (siehe «Interaktionen»), sollte das Nutzen Risiko Verhältnis, inklusive der Möglichkeit eines Auftreten von Torsade de pointes abgewogen werden, bevor eine Behandlung mit Leuprorelinacetat begonnen wird.
Im Zusammenhang mit der Anwendung von GnRH-Agonisten wurde darüber hinaus bei Männern über ein erhöhtes Risiko für einen plötzlichen Herztod berichtet.
Bei ausgeprägten lokalen Reaktionen kann die Resorption von Leuprorelin aus dem Depot beeinträchtigt sein. In einem solchen Fall sollte der Hormonspiegel bestimmt werden.
Prostatakarzinom:
Während der ersten Wochen der Behandlung mit Lucrin Depot 3 Monate kommt es zu einem kurzfristigen Anstieg des Serumtestosteronspiegels, was zu einer vorübergehenden Verschlechterung der Krankheitssymptome führen kann, wie Zunahme von Knochenschmerzen (können symptomatisch behandelt werden), Muskelschwäche in den Beinen oder Lymphödeme.
In Einzelfällen wurde über Harnwegsobstruktionen und Kompressionen der Wirbelsäule berichtet, die zur Paralyse (eventuell mit fatalem Ausgang) führen können. Patienten mit drohenden neurologischen Komplikationen, Wirbelsäulenmetastasen oder Harnwegsobstruktion sollten daher während der ersten Behandlungswochen unter ständiger, möglichst stationärer Überwachung stehen.
Die anfängliche Zunahme der Beschwerden bildet sich üblicherweise spontan zurück, ohne dass Lucrin Depot 3 Monate abgesetzt werden muss. Nachfolgend kommt es zu Symptomen des Testosteronentzugs (vgl. «Unerwünschte Wirkungen»).
Um ein Escape in der 2. Hälfte des Behandlungsintervalls auszuschliessen, insbesondere wenn die erwartete klinische und biochemische Wirkung nicht erreicht scheint (z.B. bei Besserung der Kastrationsnebenwirkungen oder Hinweisen auf Tumorprogression), sind periodische Bestimmungen der Serum-Testosteronspiegel und des prostataspezifischen Antigens anzuraten.
Nach chirurgischer Kastration bewirkt Lucrin keine weitere Absenkung des Testosteronspiegels.
Endometriose und Mammakarzinom:
Die Behandlung der Endometriose und des Mammakarzinoms mit Lucrin Depot beruht auf einer Suppression der weiblichen Geschlechtshormone. In der Initialphase der Therapie kommt es zu einem kurzfristigen Anstieg des Serumöstradiols mit nachfolgendem Abfall auf Werte, wie sie üblicherweise in der Postmenopause vorliegen. Während der Initialphase kann es daher zu einer Zunahme der klinischen Symptome kommen. Bei adäquater Dosierung klingen diese im Verlauf der weiteren Behandlung ab.
In den meisten Fällen kommt es in den ersten Wochen der Behandlung zu einer vaginalen Blutung. Bei der Therapie von submukösen uterinen Leiomyomen wurde über Fälle schwerer vaginaler Blutungen berichtet, die medikamentöse oder operative Interventionen erforderlich machten.
Eine sichere Anwendung von Leuprorelinacetat in der Schwangerschaft wurde klinisch nicht nachgewiesen. Vor dem Beginn einer Behandlung mit Lucrin Depot 3 Monate ist es empfehlenswert, die Patientin auf eine mögliche Schwangerschaft zu untersuchen. Leuprorelinacetat ist kein Kontrazeptivum. Falls eine Empfängnisverhütung erforderlich ist, sollten nicht-hormonale Kontrazeptionsmethoden verwendet werden. Ein späterer Eintritt einer Schwangerschaft ist aufgrund der Senkung der Sexualhormonspiegel nicht zu erwarten.
Mammakarzinom:
Zu Therapiebeginn führt Leuprorelinacetat, wie andere GnRH-Analoga, zu einer kurzzeitigen Erhöhung des Serumöstradiols und damit zur möglichen Stimulation des Tumorwachstums. Bei einigen Patientinnen mit metastasierendem Mammakarzinom kann es dadurch zu einer vorübergehenden Verstärkung der tumorbedingten Symptome, namentlich zur Zunahme von Schmerzen bei Skelettmetastasen (sogenanntes «tumor flare»), sowie zu einer Hyperkalzämie kommen. Entsprechend ist eine engmaschige Überwachung der Patientinnen, insbesondere in den ersten 4 Wochen der Behandlung, angezeigt.
In der adjuvanten Behandlung des Mammakarzinoms ist das Risiko einer Stimulierung des Tumors gross, wenn die ovarielle Suppression nicht über die ganze Behandlungsdauer aufrechterhalten bleibt. Deswegen soll bei Verdacht auf ungenügende hormonelle Suppression eine Östrogenbestimmung durchgeführt werden.
Pseudotumor cerebri / idiopathische intrakranielle Hypertonie:
Bei mit Leuprorelinacetat behandelten Patienten wurde vom Auftreten von Pseudotumor cerebri (PTC) / idiopathischer intrakranieller Hypertonie berichtet. Die Patienten sollen auf Anzeichen und Symptome von PTC überwacht werden, einschliesslich Kopfschmerzen, Papillenödem, verschwommenes Sehen, Doppeltsehen, Sehverlust, Schmerzen hinter den Augen oder bei Augenbewegung, Tinnitus, Schwindel und Übelkeit. Die Patienten sollen zu einem Ophthalmologen überwiesen werden, um die Diagnose des Papillenödems zu bestätigen. Falls ein PTC bestätigt wurde, soll der Patient gemäss den bestehenden Behandlungsrichtlinien therapiert werden und die Anwendung mit Leuprorelinacetat soll dauerhaft abgebrochen werden.
Anwendung bei Kindern
Die Sicherheit und Wirksamkeit von Lucrin Depot 3 Monate bei Kindern wurden nicht untersucht.
Dieses Arzneimittel enthält weniger als 1 mmol Natrium (23 mg) pro Zweikammerspritze, d.h. es ist nahezu «natriumfrei».
InteraktionenBis jetzt keine bekannt.
Es wurden keine Interaktionsstudien mit Lucrin Depot 3 Monate durchgeführt. Der Metabolismus von Leuprorelinacetat lässt Interaktionen jedoch selten erwarten, da Leuprorelin überwiegend durch Peptidasen und nicht über das Cytochrom P 450 abgebaut wird und nur eine geringe Plasmaproteinbindung von ca. 46 % aufweist.
Prostatakrebs
Da eine Androgen-Deprivation eine QT Verlängerung verursachen kann, sollte die gleichzeitige Verabreichung von Leuprorelinacetat mit Arzneimitteln, welche eine Torsade de pointes hervorrufen können, wie Antiarrhythmika der Klasse IA (z.B. Quinidin, Disopyramid) oder der Klasse III (z.B. Amiodaron, Sotalol, Dofetilid, Ibutilid), Methadon, Moxifloxacin, Antipsychotika, etc., sorgfältig abgewogen werden. Siehe «Warnhinweise und Vorsichtsmassnahmen», Effekte auf das QT-Intervall.
Schwangerschaft, StillzeitTierstudien haben unerwünschte Effekte auf den Fötus gezeigt, und es existieren keine Humanstudien.
Lucrin Depot 3 Monate ist bei Schwangeren oder Frauen, die während der Behandlung schwanger werden, kontraindiziert, da die Möglichkeit existiert, dass die Änderungen der Hormonspiegel zu einem spontanen Abort des Fötus führen.
Eine potentielle Schwangerschaft sollte vor Therapiebeginn ausgeschlossen werden (siehe «Kontraindikationen»).
Lucrin Depot 3 Monate sollte während der Stillzeit nicht verabreicht werden, da nicht bekannt ist, ob Leuprorelin in die Muttermilch ausgeschieden wird.
Wirkung auf die Fahrtüchtigkeit und auf das Bedienen von MaschinenLucrin kann das Reaktionsvermögen herabsetzen, so dass die Fähigkeit zur aktiven Teilnahme am Strassenverkehr oder zum Bedienen von Maschinen beeinträchtigt werden könnte. Dies gilt in verstärktem Mass im Zusammenhang mit Alkohol.
Unerwünschte WirkungenEine Therapie mit Leuprorelinacetat führt anfangs zu einem kurzfristigen Anstieg der Serumtestosteron- bzw. Serumöstradiolspiegel. Nachfolgend kommt es zu Symptomen des Hormonentzugs.
Die häufigsten unerwünschten Wirkungen bei Erwachsenen unter einer Behandlung mit Leuprorelinacetat sind Vasodilatation/Hitzewallungen, vermehrtes Schwitzen, Gewichtsveränderungen und verringerte Libido sowie bei Männern Potenzstörungen.
Nachfolgend werden die unerwünschten Wirkungen nach Organsystem angegeben, welche in den klinischen Studien und während der Marktbeobachtung unter Behandlung mit Leuprorelinacetat-Depotpräparaten beobachtet wurden. Die Häufigkeiten sind dabei wie folgt definiert: sehr häufig ≥1/10; häufig ≥1/100 bis <1/10; gelegentlich ≥1/1'000 bis <1/100; selten ≥1/10'000 bis <1/1'000; sehr selten <1/10'000; nicht bekannt: ausschliesslich aus Meldungen während der Marktüberwachung, genaue Häufigkeit kann nicht angegeben werden.
Allgemein:
Infektionen und parasitäre Erkrankungen
Häufig: Harnwegsinfekt, Pharyngitis
Gelegentlich: Rhinitis
Nicht bekannt: Pneumonie
Gutartige, bösartige und nicht spezifizierte Neubildungen (einschl. Zysten und Polypen)
Nicht bekannt: Hautkrebs
Erkrankungen des Blutes und des Lymphsystems
Häufig: Anämie
Nicht bekannt: Erhöhung oder Erniedrigung der Leukozytenwerte, erniedrigte Thrombozytenzahl, verlängerte Prothrombinzeit, verlängerte partielle Thromboplastinzeit
Erkrankungen des Immunsystems
Gelegentlich: Überempfindlichkeitsreaktionen
Nicht bekannt: anaphylaktische Reaktionen
Endokrine Erkrankungen
Nicht bekannt: Struma, Hypophyseninfarkt (vgl. «Warnhinweise und Vorsichtsmassnahmen»)
Stoffwechsel- und Ernährungsstörungen
Sehr häufig: Gewichtszunahme (bei Frauen: 80 %, bei Männern 22 %), gesteigerter Appetit (bei Frauen bis zu 33 %), Gewichtsabnahme (bei Frauen bis zu 25 %)
Häufig: Anorexie, Hyperglykämie
Gelegentlich: Dehydratation, Hyopoglykämie
Nicht bekannt: Hypokaliämie, Harnstofferhöhung, Kreatininerhöhung, Hypercalciämie, Hyperphosphatämie, Hypoproteinämie, Hyperurikämie, Hyperlipidämie, Diabetes mellitus
Psychiatrische Erkrankungen
Sehr häufig: verringerte Libido (bei Männern: 47 %, bei Frauen: 11 %), emotionale Labilität (bei Frauen: 24 %), Nervosität (bei Frauen bis zu 22 %), Stimmungsschwankungen (bei Frauen bis zu 20 %), Schlaflosigkeit (bei Frauen: 19 %), Depression (bei Frauen: 18 %)
Häufig: Schlafstörungen, Angst
Gelegentlich: Wahnvorstellungen
Nicht bekannt: gesteigerte Libido, Lethargie, Suizidalität
Erkrankungen des Nervensystems
Sehr häufig: Kopfschmerzen (bei Frauen bis zu 38 %), Schwindel (bei Frauen bis zu 15 %)
Häufig: Schläfrigkeit, Tremor, Parästhesien, Hypästhesien, Synkope
Gelegentlich: Geschmacksstörungen, Amnesie
Nicht bekannt: Krampfanfälle, periphere Neuropathie, Neuromyopathie, transitorische ischämische Attacke, Apoplexie, Paralyse, Bewusstseinsverlust, Pseudotumor cerebri / idiopathische intrakranielle Hypertonie
Augenerkrankungen
Häufig: Sehstörungen (z.B. Verschwommensehen), Amblyopie
Nicht bekannt: trockene Augen
Erkrankungen des Ohrs und des Labyrinths
Häufig: Tinnitus, Ohrenschmerzen
Nicht bekannt: beeinträchtigtes Hörvermögen
Herzerkrankungen
Häufig: Tachykardie, Arrhythmien, ventrikuläre Extrasystolen, Angina pectoris
Gelegentlich: Bradykardie, Herzinsuffizienz
Nicht bekannt: Herzgeräusche, EKG-Veränderungen (z.B. Zeichen einer myokardialen Ischämie), AV-Block, Myokardinfarkt, QT-Verlängerungen (siehe «Warnhinweise und Vorsichtsmassnahmen» und «Interaktionen»)
Gefässerkrankungen
Sehr häufig: Vasodilatation (Frauen: 83 %, Männer: 57 %), Hitzewallungen (Frauen: 83 %, Männer: 52 %)
Häufig: Thrombophlebitis, Hypertonie, Lymphödem
Nicht bekannt: Hypotonie, Thrombose, Lungenembolie
Erkrankungen der Atemwege, des Brustraums und Mediastinums
Häufig: Husten, Dyspnoe, Epistaxis, Hämoptysen
Nicht bekannt: Pleurareiben, Pleuraerguss, Lungeninfiltration, Lungenfibrose, interstitielle Lungenerkrankung
Erkrankungen des Gastrointestinaltrakts
Sehr häufig: Übelkeit (bei Frauen bis zu 14 %)
Häufig: Mundtrockenheit, Flatulenz, Obstipation, Diarrhoe, Erbrechen, Gastritis
Nicht bekannt: Dysphagie, gastroduodenale Ulcera, gastrointestinale Blutung, rektale Polypen
Leber- und Gallenerkrankungen
Sehr häufig: Erhöhung der AST (bei Männern bis zu 20 %)
Häufig: Leberfunktionsstörungen (z.B. Erhöhung der ALT, Erhöhung der Gamma-GT)
Nicht bekannt: schwere Leberschädigung, Hyperbilirubinämie, Ikterus
Erkrankungen der Haut und des Unterhautgewebes
Häufig: trockene Haut, Hautausschlag (einschliesslich makulopapulösem Exanthem), Pruritus, Urtikaria, Haarveränderungen (insbesondere Zu- oder Abnahme der Körperbehaarung), Alopezie, Ekchymosen, Pigmentierungsstörungen
Gelegentlich: Photosensitivitätsreaktionen
Nicht bekannt: Erythema multiforme, Dermatitis, bullöse Dermatitis, exfoliative Dermatitis, Stevens-Johnson-Syndrom, toxische epidermale Nekrolyse, Hautläsionen, Knötchen
Skelettmuskulatur-, Bindegewebs- und Knochenerkrankungen
Sehr häufig: Knochenschmerzen (bei Männern bis zu 14 %), Arthralgien (bei Frauen bis zu 14 %), Rückenschmerzen (bei Frauen bis zu 14 %), Muskelschwäche (bei Männern bis zu 11 %)
Häufig: Myalgie, Muskelkrämpfe, Arthropathie
Nicht bekannt: Reduktion der Knochendichte, Knochenschwellung, Tenosynovitis, ankylosierende Spondylitis
Bei einer Langzeittherapie (6-12 Monate) wurde darüber hinaus über eine Osteoporose berichtet.
Erkrankungen der Nieren und Harnwege
Sehr häufig: Nykturie (bei Männern bis zu 17 %)
Häufig: Pollakisurie, Dysurie, Hämaturie
Gelegentlich: Harninkontinenz
Nicht bekannt: vermehrter Harndrang, Spasmen der Harnblase, Harnwegsobstruktion
Allgemeine Erkrankungen und Beschwerden am Verabreichungsort
Sehr häufig: Indurationen an der Injektionsstelle (bei Frauen bis zu 31 %), vermehrtes Schwitzen (Frauen: 78 %; Männer: bis 42 %), Asthenie (bei Frauen bis zu 14 %), Müdigkeit (bei Männern bis zu 13 %), Erythem an der Injektionsstelle (bei Männern bis zu 13 %), Schmerzen an der Injektionsstelle (bei Frauen bis zu 13 %), periphere Ödeme (bei Männern bis zu 12,5 %)
Häufig: andere Reaktionen an der Injektionsstelle (wie Juckreiz, Schwellung, Hämatom, Entzündung, Abszess), vermehrtes Durstgefühl, Unwohlsein, Schweissausbrüche, Nachtschweiss, Fieber, Schüttelfrost, Ödeme, Brustschmerzen
Nicht bekannt: Fibrose im Beckenbereich, Nekrose an der Injektionsstelle
Männer:
In klinischen Studien oder in der PMS wurden bei Männern darüber hinaus die folgenden unerwünschten Wirkungen beobachtet:
Infektionen und parasitäre Erkrankungen
Häufig: Bronchitis
Gelegentlich: Candidose, Pilzinfektion auf der Haut
Stoffwechsel- und Ernährungsstörungen
Häufig: Hypercalciämie, Erhöhung der AP
Erkrankungen des Nervensystems
Gelegentlich: Gangstörung
Herzerkrankungen
Nicht bekannt: plötzlicher Herztod (vgl. «Warnhinweise und Vorsichtsmassnahmen»)
Gefässerkrankungen
Sehr häufig: Flush (34 %)
Gelegentlich: periphere Zirkulationsstörung
Nicht bekannt: orthostatische Hypotonie
Erkrankungen der Atemwege, des Brustraums und Mediastinums
Häufig: Asthma bronchiale, akutes Lungenödem, Lungenemphysem
Gelegentlich: chronisch-obstruktive Lungenerkrankung
Leber- und Gallenerkrankungen
Sehr häufig: Erhöhung der LDH im Serum (29 %)
Gelegentlich: hepatozelluläre Schädigungen, cholestatische Hepatitis
Nicht bekannt: nicht-alkoholische Fettlebererkrankung
Erkrankungen der Haut und des Unterhautgewebes
Nicht bekannt: Haarwuchsstörungen
Skelettmuskulatur-, Bindegewebs- und Knochenerkrankungen
Häufig: Schmerzen in den Extremitäten
Erkrankungen der Nieren und Harnwege
Gelegentlich: Miktionsstörungen, Harnretention, Polyurie
Erkrankungen der Geschlechtsorgane und der Brustdrüse
Sehr häufig: Potenzstörungen (45 %)
Häufig: Gynäkomastie, Hodenatrophie, PSA-Erhöhung
Sehr selten: Spannungsgefühl oder Schmerzen in der Brust, Penisschwellung, Schmerzen in der Prostataregion
Nicht bekannt: Hodenschmerz
Allgemeine Erkrankungen und Beschwerden am Verabreichungsort
Gelegentlich: trockene Schleimhäute
Untersuchungen
Gelegentlich: Proteine im Urin, erhöhter Retikulozytenwert
Frauen:
In den meisten Fällen kommt es in den ersten Wochen der Behandlung zu einer Blutung.
In klinischen Studien oder in der PMS wurden bei Frauen über die oben genannten unerwünschten Wirkungen hinaus folgende Effekte beobachtet:
Infektionen und parasitäre Erkrankungen
Häufig: vulvovaginale Candidose, Influenza
Gelegentlich: Pyelonephritis, Furunkel
Erkrankungen des Blutes und des Lymphsystems
Gelegentlich: Lymphadenopathie, Koagulopathie
Endokrine Erkrankungen
Häufig: Thyreoiditis
Stoffwechsel- und Ernährungsstörungen
Häufig: verminderter Appetit
Psychiatrische Erkrankungen
Häufig: Unruhe, Feindseligkeit, Verwirrtheit, abnormales Denken
Gelegentlich: Apathie, Euphorie, Persönlichkeitsstörung
Erkrankungen des Nervensystems
Sehr häufig: Migräne (13 %)
Häufig: Koordinationsstörungen, Hyperkinesie, lokale Krämpfe
Gelegentlich: Ataxie
Augenerkrankungen
Häufig: Konjunktivitis
Gelegentlich: Augenschmerzen
Erkrankungen des Ohrs und des Labyrinths
Häufig: aurikuläre Schwellung
Herzerkrankungen
Häufig: Palpitationen
Bei Patientinnen, welche mit Leuprorelin behandelt wurden, wurde über Fälle von venösen und arteriellen thromboembolischen Ereignissen (wie tiefer Venenthrombose, Lungenembolie, Myokardinfarkt, transienter ischämischer Attacke und Apoplexie) berichtet. Häufig lagen jedoch gleichzeitig weitere Risikofaktoren für die Entstehung solcher Ereignisse vor (einschliesslich Komedikationen mit entsprechendem Risiko). Über einen möglichen Kausalzusammenhang mit der Anwendung von GnRH-Agonisten ist bisher keine Aussage möglich.
Erkrankungen der Atemwege, des Brustraums und Mediastinums
Gelegentlich: Dysphonie, Laryngospasmus
Erkrankungen des Gastrointestinaltrakts
Häufig: Bauchschmerzen, Dyspepsie, Gingivitis, Stomatitis, Melaena
Gelegentlich: Zahnfleischbluten
Leber- und Gallenerkrankungen
Gelegentlich: Druckempfindlichkeit der Leber
Nicht bekannt: Steatose der Leber
Erkrankungen der Haut und des Unterhautgewebes
Sehr häufig: Akne (10 %)
Häufig: Erythem, Seborrhoe, Ekzem, Hirsutismus, Nagelveränderungen
Gelegentlich: Hautverfärbungen
Skelettmuskulatur-, Bindegewebs- und Knochenerkrankungen
Häufig: Nackenschmerzen, Nackensteifigkeit, muskuloskelettale Steifigkeit, Osteoarthritis
Gelegentlich: Muskelzuckungen
Erkrankungen der Nieren und Harnwege
Häufig: Schmerzen in der Nierengegend
Erkrankungen der Geschlechtsorgane und der Brustdrüse
Sehr häufig: Vaginitis (26 %), Brustschmerzen (13 %)
Häufig: Vergrösserung der Brust, menopausale Symptome, Unterbauchschmerzen, Spannungsgefühl in der Brust, Atrophie der Brust, Fluor vaginalis, Dysmenorrhoe, Menorrhagie, Metrorrhagie, Galaktorrhoe, Dyspareunie
Gelegentlich: Induration der Brust, vaginale Blutungen, Brusttumor
Nicht bekannt: Trockenheit der Vagina, Zyklusstörungen
Allgemeine Erkrankungen und Beschwerden am Verabreichungsort
Sehr häufig: Hitzegefühl (72 %), Verschlechterung des Allgemeinzustandes (28 %)
Häufig: Reizbarkeit, generalisierte Ödeme
Gelegentlich: Gesichtsödem
Die Meldung des Verdachts auf Nebenwirkungen nach der Zulassung ist von grosser Wichtigkeit. Sie ermöglicht eine kontinuierliche Überwachung des Nutzen-Risiko-Verhältnisses des Arzneimittels. Angehörige von Gesundheitsberufen sind aufgefordert, jeden Verdacht einer neuen oder schwerwiegenden Nebenwirkung über das Online-Portal ElViS (Electronic Vigilance System) anzuzeigen. Informationen dazu finden Sie unter www.swissmedic.ch.
ÜberdosierungIntoxikationssymptome wurden bisher nicht beobachtet.
In klinischen Studien wurden bei Erwachsenen Dosen bis zu 20 mg/Tag über zwei Jahre verabreicht. Die unerwünschten Wirkungen unter dieser hohen Dosierung unterschieden sich nicht von jenen unter Applikation von 1 mg/Tag.
Im Fall einer Überdosierung sollte der Patient überwacht werden. Gegebenenfalls sind symptomatische und supportive Massnahmen unter ärztlicher Kontrolle angezeigt.
Eigenschaften/WirkungenATC-Code
L02AE02
Wirkungsmechanismus
Pharmakodynamik
Der Wirkstoff Leuprorelinacetat ist ein synthetisches Analogon des natürlich vorkommenden Gonadorelins (GnRH-Analog), das die Freisetzung der gonadotropen Hormone LH und FSH aus dem Hypophysenvorderlappen kontrolliert. Diese Hormone stimulieren ihrerseits die testikuläre und ovarielle Steroidsynthese.
Im Gegensatz zum physiologischen Gonadorelin, das pulsatil vom Hypothalamus freigesetzt wird, blockiert Leuprorelinacetat bei therapeutischer Daueranwendung die Gonadorelinrezeptoren der Hypophyse kontinuierlich und verursacht nach einer initialen kurzfristigen Stimulation deren Desensibilisierung (Down-Regulierung). Als Folge kommt es nach zwei bis vier Wochen zu einer reversiblen hypophysären Suppression der Gonadotropin-Freisetzung mit gleichzeitigem Abfall des Testosterons auf Kastrationsniveau respektive des Östrogenspiegels auf Werte nach einer Ovarektomie oder in der Postmenopause (<30 pg/ml) und zu einem Ausbleiben der Regelblutung.
Dieser Zustand mit tiefen Testosteron- bzw. Östrogenspiegeln bleibt während der gesamten Therapiedauer erhalten. Dies führt zu einer Wachstumshemmung von hormonabhängigen Tumoren wie des Prostatakarzinoms oder des Mammakarzinoms sowie von uterinem und ektopischem Endometriumgewebes. Im Verlauf der Behandlung tritt dadurch eine Besserung der Symptomatik ein.
Bei wiederholter Gabe kommt es zu einer anhaltenden Senkung des Testosteronspiegels in den Kastrationsbereich, ohne dass der Testosteronspiegel wie nach erstmaliger Injektion einen vorübergehenden Anstieg zeigt.
Nach Absetzen der empfohlenen Sechs-Monate-Therapie der Endometriose tritt die Regelblutung im Mittel nach drei Monaten wieder ein.
Klinische Wirksamkeit
Bei der Therapie des Mammakarzinoms mit GnRH-Agonisten besteht das pharmakodynamische Behandlungsziel in einer Senkung der Östradiol-Spiegel auf postmenopausale Werte (<30 pg/ml). Dies wurde in zwei klinischen Studien mit Vergleich der 1 Monats- und der 3 Monats-Depotformulierung für beide Depotformen über 24 Wochen Behandlung nachgewiesen.
In einer klinischen Phase III Studie an n=537 prä- und perimenopausalen Patientinnen mit einem Mammakarzinom (Tumorgrösse T1–3, mit positiven lokalen Lymphknoten (N+), jedoch ohne Fernmetastasen (M0)) wurde die Wirksamkeit von Lucrin Depot 3 Monate zur adjuvanten Therapie mit jener von CMF (Cyclophosphamid, Methotrexat und 5-Fluorouracil) verglichen. Die Östradiolwerte unter Lucrin Depot 3 Monate über 24 Monate sind in der folgenden Tabelle angegeben.
|
Östradiolspiegel bei Patientinnen, welche mit
Leuprorelinacetat Depot 3 Monate behandelt wurden
(Per Protokoll (PP) Population)
| |
Therapiedauer
|
Untersuchte Patientinnen
|
E2 <30 pg/ml
| |
n
|
n
|
%
| |
vor Therapiebeginn
|
139
|
37
|
26,6
| |
3 Monate
|
167
|
130
|
77,8
| |
6 Monate
|
201
|
183
|
91
| |
12 Monate
|
226
|
213
|
94,2
| |
18 Monate
|
219
|
212
|
96,8
| |
24 Monate
|
189
|
170
|
89,9
|
Klinische Studien bei Endometriose
In einer doppelblinden, vierarmigen Studie an n=201 Endometriose-Patientinnen mit mässiger bis schwerer Schmerzsymptomatik wurde über eine Behandlungsdauer von 12 Monaten eine Monotherapie mit Leuprorelin 3,75 mg alle 4 Wochen verglichen mit einer add back-Therapie mit 5 mg Norethisteronacetat (NETA)/Tag, 5 mg NETA + 0,625 mg konjugierten equinen Östrogenen (CEE) oder 5 mg NETA + 1,25 mg CEE. Eingeschlossen wurden sowohl therapienaive Patientinnen als auch Patientinnen mit Symptompersistenz oder Rezidiv nach einer chirurgischen oder medikamentösen Therapie. Neben der Wirksamkeit (Verbesserung der Schmerzsymptomatik auf der Biberoglu & Behrman-Skala) wurden in dieser Studie auch die Knochendichte (BMD, mit der DEXA-Methode) nach 6 und 12 Monaten (sowie bis 24 Monate nach Therapieende) sowie vasomotorische Symptome untersucht. In allen vier Behandlungsarmen kam es zu einer signifikanten Verbesserung der Endometriose-Symptomatik gegenüber Baseline, wobei die Verbesserung unter add back-Therapie mit 5 mg NETA + 1,25 mg CEE etwas geringer ausgeprägt war als in den anderen Gruppen. Patientinnen unter add back-Therapie mit 5 mg NETA/Tag berichteten signifikant seltener über Hitzewallungen als Patientinnen unter Leuprorelin-Monotherapie (60 % vs. 88 %, p<0,05). Die BMD, welche allerdings nur als Sekundärendpunkt untersucht wurde, nahm unter der Leuprorelin-Monotherapie innerhalb von 12 Monaten um 6,3 % ab, während sie in den add back-Gruppen weitgehend stabil blieb (5 mg NETA: Reduktion um 0,9 %; 5 mg NETA + 0,625 mg CEE: Reduktion um 0,2 %; 5 mg NETA + 1,25 mg CEE: Zunahme um 0,6 %). Am Ende des 24-monatigen Follow up hatten die Patientinnen in den add back-Gruppen die Ausgangswerte wieder erreicht, während im Monotherapiearm die BMD noch um rund 1 % niedriger lag als vor Therapiebeginn.
In einer zweiten, offenen, unkontrollierten Studie an n=136 Endometriose-Patientinnen wurde der Einfluss einer 12-monatigen GnRH-Behandlung mit add back-Therapie mit 5 mg NETA/Tag auf die Knochendichte als Primärendpunkt untersucht. Bis zum Therapieende kam es zu einer Abnahme der BMD um 1 %. Am Ende des 12-monatigen Follow up waren die Ausgangswerte der BMD wieder erreicht.
Kastrationsresistentes Prostatakarzinom
In klinischen Studien konnte bei Patienten mit metastasiertem kastrationsresistentem Prostatakarzinom der Nutzen einer zusätzlichen Wirkstoffgabe wie z.B. den Androgenachse-Inhibitoren Abirateronacetat und Enzalutamid, den Taxanen Docetaxel und Cabazitaxel sowie dem Radiopharmakon Ra-223 zusätzlich zu GnRH Agonisten wie Leuprorelinacetat gezeigt werden.
PharmakokinetikAbsorption
Leuprorelinacetat wird nach Injektion von Lucrin Depot 3 Monate kontinuierlich aus dem Milchsäurepolymer über einen Zeitraum von drei Monaten freigesetzt. Das Copolymer wird dabei wie chirurgisches Nahtmaterial resorbiert.
Die Bioverfügbarkeit nach subkutaner Verabreichung unterscheidet sich nicht signifikant von jener nach intramuskulärer Verabreichung.
Spitzenspiegel von 22-36 ng/ml werden innerhalb von 3-4 Stunden nach der Injektion erreicht. 7-14 Tage nach Injektion wird das Plateau erreicht. Danach nimmt der Plasmaspiegel allmählich ab und liegt 4 Wochen nach Injektion bei 0,23-0,26 ng/ml. 12 Wochen nach Injektion liegt die Plasmakonzentration bei ca. 0,17 (+/- 0,08) ng/ml.
Leuprorelinacetat wird nach oraler Verabreichung nicht resorbiert.
Distribution
Bei gesunden Männern beträgt das mittlere Verteilungsvolumen im Steady-State nach intravenöser Bolusinjektion 27 l. Die Plasmaproteinbindung in vitro beträgt zwischen 43 – 49 %.
Metabolismus
In Tierstudien wurde 14C-markiertes Leuprorelin zu folgenden kleineren, inaktiven Peptiden metabolisiert: Pentapeptid (Metabolit-I), Tripeptide (M-II und M-III), Dipeptid (M-IV). Die Peptidfragmente werden eventuell noch weiter abgebaut.
Zwei bis 6 Stunden nach Injektion von Leuprorelinacetat Depot Suspension wurden bei 5 Patienten mit Prostatakarzinom maximale Plasmakonzentrationen des Hauptmetaboliten (M-I) gemessen. Dies entspricht rund 6 % der maximalen Leuprorelinacetat Konzentration. Eine Woche nach Injektion betrug die mittlere Plasmakonzentration von M-I rund 20 % der mittleren Leuprorelin Konzentration.
Elimination
Nach Verabreichung von Leuprorelinacetat Depot Suspension 3,75 mg an 3 Patienten wurde über die Dauer von 27 Tagen weniger als 5 % der verabreichten Dosis unverändert resp. in Form von M-I in den Urin ausgeschieden.
Die mittlere Serum-Clearance nach intravenöser Bolusinjektion von 1 mg Leuprorelin beträgt bei gesunden Männern 7,6 l/h mit einer terminalen Eliminationshalbwertszeit von rund 3 Stunden (2-Kompartiment-Modell).
Kinetik spezieller Patientengruppen
Kinder und Jugendliche: Es liegen keine Daten zur Pharmakokinetik von Lucrin Depot 3 Monate (11,75 mg) bei Kindern und Jugendlichen vor.
Ältere Patienten: Die Pharmakokinetik bei älteren Patienten unterscheidet sich nicht in relevanter Weise von jener bei jüngeren Erwachsenen.
Leber- und Niereninsuffizienz: Bei Patienten mit eingeschränkter Nierenfunktion wurden teilweise höhere Serumspiegel an Leuprorelinacetat gemessen, bei Patienten mit eingeschränkter Leberfunktion dagegen verminderte Werte. Die klinische Signifikanz dieser Beobachtung ist unbekannt.
Präklinische DatenMutagenität
Untersuchungen zur Mutagenität (an Bakterien- und Säugetierzellen) haben keine Zeichen eines mutagenen Potentials von Leuprorelin erkennen lassen.
Karzinogenität
In Karzinogenitätsstudien wurden Mäuse und Ratten während 2 Jahren mit Leuprorelin behandelt. Nach 24 Monaten konnte bei Ratten nach subkutaner Verabreichung von 0,6 bis 4 mg/kg eine dosisabhängige Zunahme von gutartigen hypophysären Hyperplasien und Adenomen beobachtet werden. Weiterhin wurde eine dosisunabhängige Zunahme von Adenomen der Pankreas-Inselzellen bei weiblichen Ratten und der Hodenzwischenzellen bei männlichen Tieren festgestellt. Bei Mäusen verursachten Dosierungen bis zu 60 mg/kg, welche ebenfalls während 2 Jahren verabreicht wurden, keine durch Leuprorelin induzierte Tumore oder Anomalien der Hypophyse.
Bei Patienten, welche subkutan mit Leuprorelin während 3 Jahren mit täglich 10 mg oder während 2 Jahren mit täglich 20 mg behandelt wurden, traten ebenfalls keine hypophysären Anomalien auf.
Reproduktionstoxizität
Untersuchungen mit subkutaner Verabreichung von Leuprorelin an Ratten bei Dosen bis 10 µg/kg und an Kaninchen bei Dosen bis 1 µg/kg haben keine Hinweise auf ein teratogenes Potential ergeben. Embryotoxische/embryoletale Wirkungen wurden bei der Ratte bei einer Dosis von 10 µg/kg und beim Kaninchen bei Dosen über 0,1 µg/kg beobachtet.
Sonstige HinweiseInkompatibilitäten
Bis jetzt keine bekannt.
Beeinflussung diagnostischer Methoden
Bei Frauen führt die Verabreichung von Leuprorelinacetat Depot zur Supression des hypophysär-gonadalen Regelkreises. Üblicherweise wird innerhalb von 3 Monaten nach Absetzen von Leuprorelinacetat die normale Funktion wieder erhalten. Aus diesem Grund können diagnostische Testergebnisse hypophysär gonadotroper und gonadaler Funktionen während bis zu 3 Monate nach Absetzen von Leuprorelinacetat irreführend sein.
Haltbarkeit
Das Arzneimittel darf nur bis zu dem auf der Packung mit «EXP» bezeichneten Datum verwendet werden.
Bei Auftreten einer Verfärbung der Trockensubstanz und/oder Trübung des Lösungsmittels dürfen diese nicht mehr verwendet werden.
Besondere Lagerungshinweise
Ausser Reichweite von Kindern aufbewahren.
Bei Raumtemperatur (15-25 °C) lagern. Nicht einfrieren. In der Originalverpackung aufbewahren, um den Inhalt vor Licht zu schützen.
Hinweise für die Handhabung
Zubereitung der Suspension:
Zweikammerspritze: Zur Suspendierung darf ausschliesslich das beigefügte Lösungsmittel verwendet werden. Durch langsames Drücken des Kolbens in den Zylinder wird das Lösungsmittel in die Kammer mit den Mikropartikeln entleert. Durch sorgfältiges Durchmischen entsteht eine homogene, milchige Suspension.
Wird die Suspension nicht unmittelbar nach der Zubereitung appliziert, muss sie verworfen werden, da Lucrin Depot 3 Monate keine Konservierungsstoffe enthält.
Zur Injektion wird eine Nadel von 23 Gauge (z.B. 0,6 x 25 mm) empfohlen.
Zulassungsnummer54231 (Swissmedic).
Packungen1 Zweikammerspritze und 1 Alkoholtupfer (A)
ZulassungsinhaberinAbbVie AG, 6330 Cham
Stand der InformationMai 2025
Lucrin Depot Zweikammer-Spritze
Für eine korrekte Handhabung der vorgefüllten Lucrin Depot Zweikammer-Spritze, bitten wir Sie, die folgenden Anweisungen zu lesen und genau zu befolgen.
|
|
|
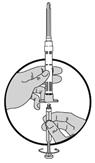
1.Schrauben Sie den weissen Kolben zur Vorbereitung der Injektion in den Endstopfen, bis dieser anfängt, sich zu drehen.
Vergessen Sie nicht, den Luer-Lock-Mechanismus zu spannen, indem Sie die Nadel im Uhrzeigersinn drehen, bis diese sich nicht mehr bewegen lässt. Überdrehen Sie diese nicht!
| |
|
|
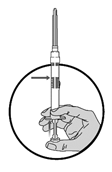
2.Halten Sie die Spritze senkrecht nach oben und setzen Sie das Verdünnungsmittel frei, indem Sie den Kolben langsam schieben, bis der erste Stopfen die blaue Linie in der Mitte des Zylinders erreicht hat.
| |
|
|
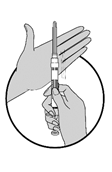
3.Schwenken (nicht schütteln) Sie die Spritze, damit sich die Teilchen zu einer homogenen Suspension vermischen können. Diese Suspension hat ein milchiges Aussehen.
4.Falls die Mikrokugeln (Teilchen) am Stopfen hängen bleiben, klopfen Sie die Spritze leicht gegen Ihren Finger.
| |
|
|
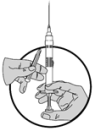
5.Nehmen Sie dann den Nadelschutz ab und schieben Sie den Kolben weiter vor, um die Luft aus der Spritze zu entfernen.
| |
|
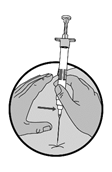
|
6.Injizieren Sie den gesamten Inhalt der Spritze sofort nach der Zubereitung intramuskulär oder subkutan wie bei einer normalen Injektion. Die Suspension sedimentiert sehr schnell, daher sollte die Lucrin Suspension sofort verwendet werden.
| |
|
ACHTUNG: Angesaugtes Blut würde unmittelbar unter der Luer-Lock-Verbindung sichtbar werden.
7.Nach Verwendung Spritze fachgerecht entsorgen.
|
|





